We have incredible news to share! Massachusetts-based bluebird bio, a leader in the research and treatment of cancer and severe genetic diseases, just received FDA “Breakthrough Therapy” designation for their Lenti-D™ gene therapy for patients with cerebral adrenoleukodystrophy (CALD). This designation will allow for expedited development and review of the treatment protocol, meaning it is …Read More
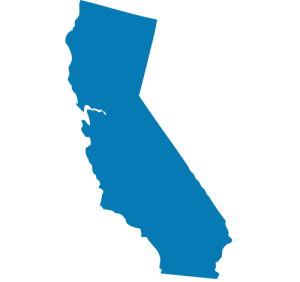
California Begins Newborn Screening for ALD
California Begins Newborn Screening for ALD It’s official, California is testing their babies for ALD, at the rate of 1,400 births per day! In addition to this screening, they will also retroactively screen blood samples from newborns born on or after February of this year. This is a huge milestone for the ALD community, and the California Department of …Read More
20th Annual Hammerfest was an Amazing Success
Hammerfest 2016 was a great success, with beautiful weather and smiles all around! We celebrated 20 years of great races for great causes, The Myelin Project and Brian's Hope, both in support of the ALD community. We want to extend a heartfelt thank you to our hosts at the Owenego, the triathletes, our steadfast volunteers, …Read More
Press Conference on ALD Newborn Screening Success Held in Connecticut
Check out the article featured in the New Haven Register about the press conference announcement and the journey it has taken to achieve this accomplishment. We are so blessed to have the support of our local community behind us! Click here to read the article.
Gene Therapy Stabilizes Disease in Cerebral ALD
Exciting news for the ALD Community! Our friends at Bluebird Bio Inc. have sponsored the Starbeam Study to study gene therapy for boys with ALD who don’t have a bone marrow transplant sibling match. Autologous hematopoietic stem cell (HSC) gene therapy may offer a safe and effective alternative to allogeneic bone marrow transplant for patients …Read More
- 1
- 2
- 3
- …
- 7
- Next Page »